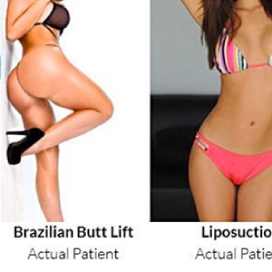
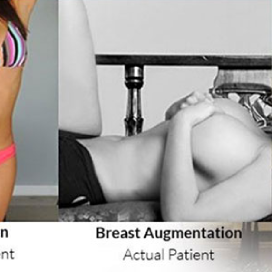
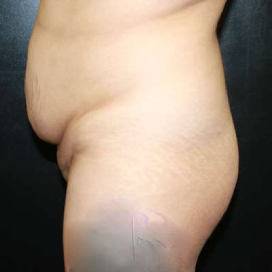
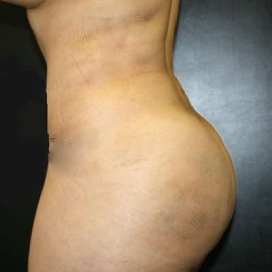
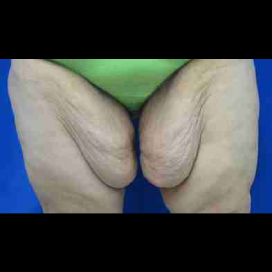
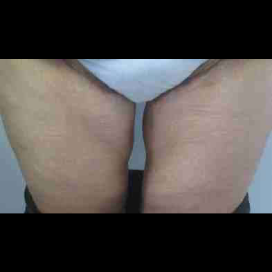
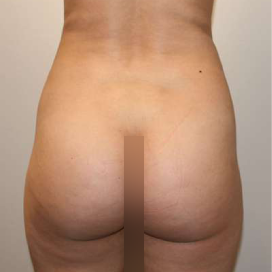
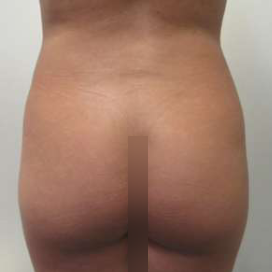
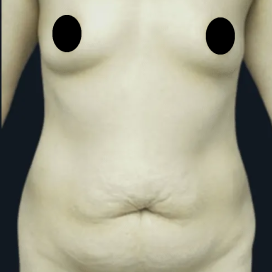
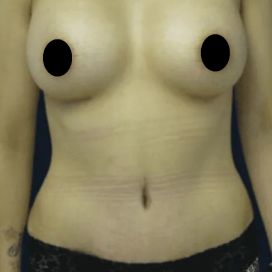
Chapter 7 Scar Revision in Plastic Surgery
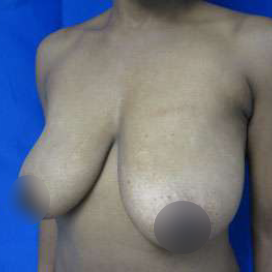
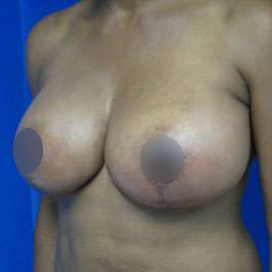
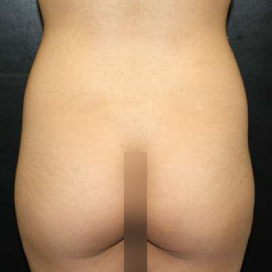
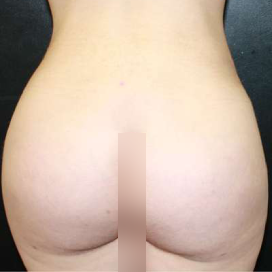
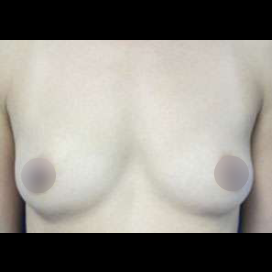
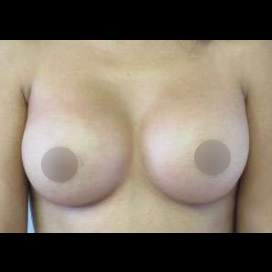
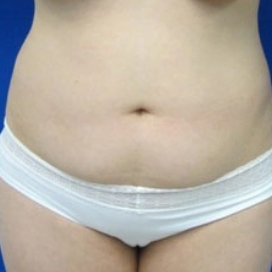
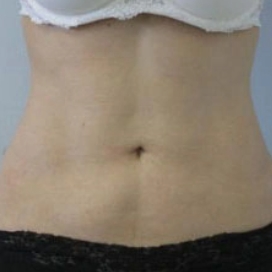
Introduction Scars are the inevitable result of any surgical procedure. The goal of the Plastic Surgeon is to generate a scar that does not impair form or function to any significant degree. Attention to detail and precision in wound creation and closure maximize the opportunity for producing an excellent scar […]
Read More